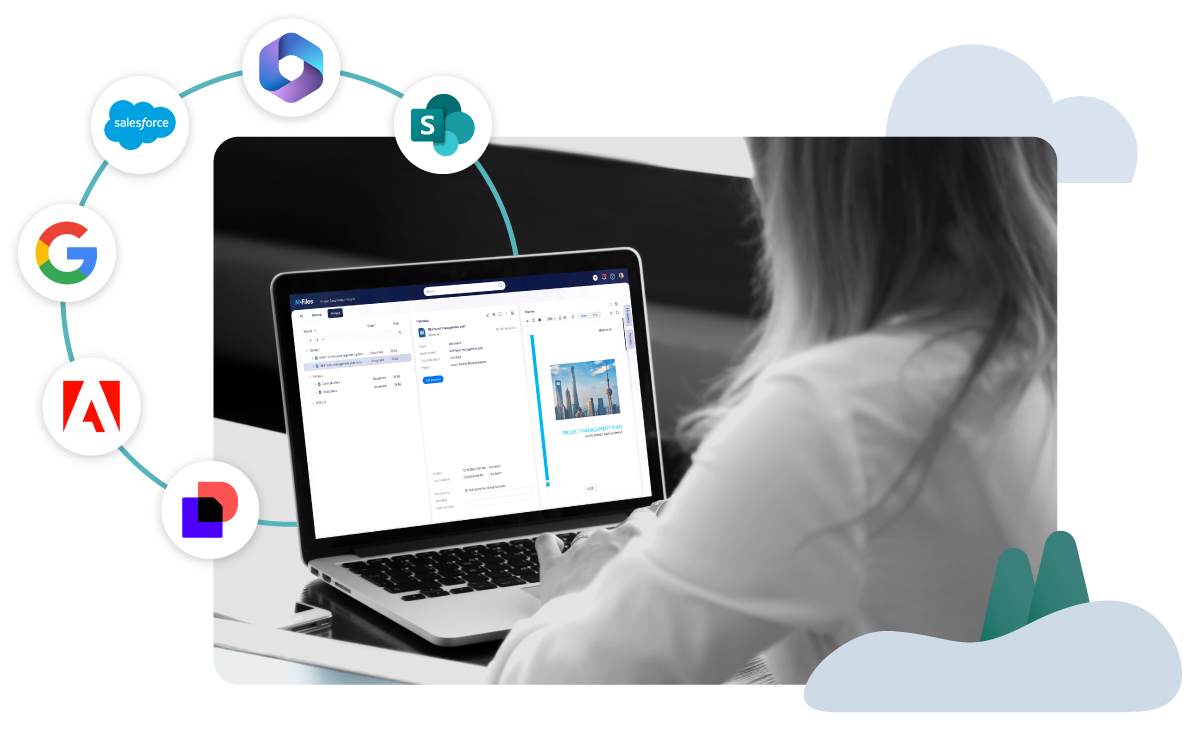
CROs Increase Productivity and Improve Control Through Accessibility and Automation of Documentation
Revolutionize and simplify how your firm manages information with the leading Knowledge Work Automation Platform. Automate workflows, find and organize client files and information instantly, fortify your security and compliance environment all while leveraging M-Files powered GenAI. Get connected with a scalable solution.
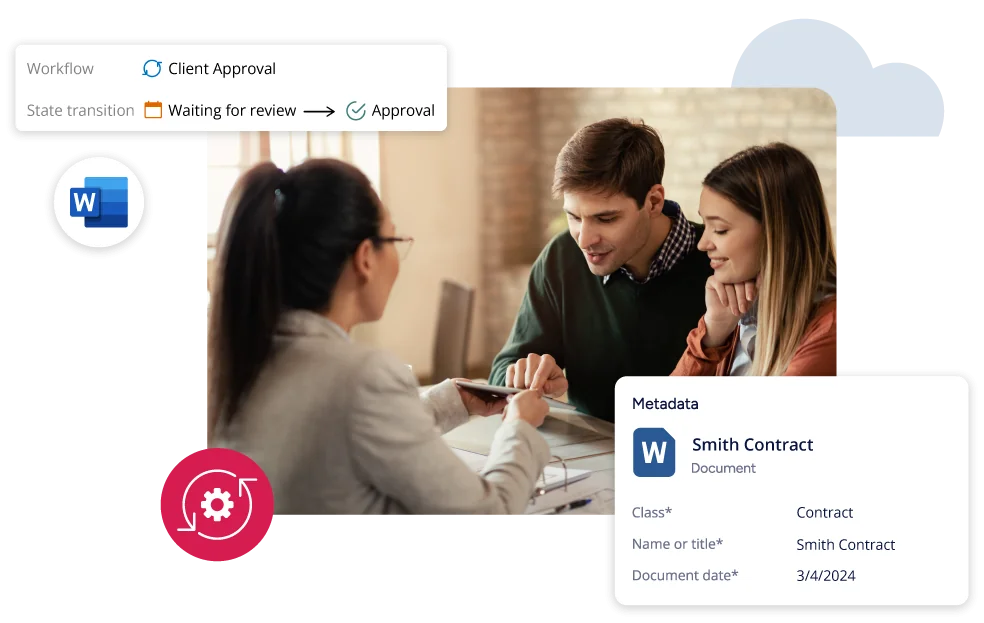
Unlock Profit and Growth while Reducing Risk
Improve productivity, quality and profitability for contract research organizations with better document management.

Automate Documentation of SOPs and CAPAs
- Automate SOPs and resolve CAPAs efficiently through programmed workflows and alerts that provide visibility to deviations from standards
- Meet schedule milestones in conformance with SOPs through review workflows, timelines, reminders, and notifications of delays
- Control quality with workflows, security controls, records management, and approvals automatically implementing your policies
- Increase growth capacity by automating routine tasks so your firm can handle more studies without staff additions
- Improve CRA recruitment and retention by equipping them with tools that reduce their administrative tasks and create more time for science
- Accelerate staff onboarding through templates and workflows that enable them to quickly execute SOPs and handle CAPAs

Reduce Business Risk by Ensuring Compliance
- Ensure content security by making documents and data sets available to the right people no matter where or by whom they are stored
- Improve blinding by ensuring that users have zero data visibility unless and until they are identified as approved for access
- Provide evidence of operational oversight that inspires confidence in sponsors and meets regulatory requirements
- Maintain Trial Master Files in conformance with regulatory requirements through automated SOP execution
- Streamline audits by showing SOP and CAPA controls and record-level tracking of how and when data was generated, approved, and accessed

Streamline Information Management for Contract Research Organizations (CROs)
- Enhance researcher productivity by streamlining complex documentation tasks, allowing CROs to focus on clinical trials and research development.
- Ensure document accuracy through consistent application of master data and alerts that flag missing or outdated information.
- Maximize CRM value by making critical research documents easily accessible through primary operational interfaces.
- Improve client acquisition by leveraging past research insights to strengthen proposals and enhance future collaborations.

Strengthen Sponsor Relationships Through Collaborative Workspaces
- Centralize sponsor communications, including inputs, outputs, reviews, and collaborations in a single, secure workspace per project
- Ensure regulatory conformance by tracking when physicians at investigative sites receive, open, and act on critical communications
- Avoid project delays through CRO/sponsor workflows, alerts on open tasks, and the ability to collaborate on information in real time
- Avoid losing clinical data that might be overlooked by ensuring version status is not incorrectly coded or miscommunicated
- Track information exchange between investigative sites and sponsors, including acknowledgement of receipt
Work Smarter with M-Files
The M-Files Impact
Up to 294% return on investment with low cost of initial entry and fast time to value.
See How The Most Effective Organizations Leverage M-Files

Fingrid
M-Files delivers an agile, scalable and intuitive information management solution for Finnish electricity transmission service provider.

Manzil Healthcare
In this case study, discover how Manzil, a healthcare services provider increased efficiency and reduced their dependency on paper-based processes with document management from M-Files.
The Smarter Way to Work with Automation and AI